Scientists on the McGovern Institute for Mind Analysis at MIT and the Broad Institute of MIT and Harvard have re-engineered a compact RNA-guided enzyme they present in micro organism into an environment friendly, programmable editor of human DNA.
The protein they created, known as NovaIscB, may be tailored to make exact modifications to the genetic code, modulate the exercise of particular genes, or perform different modifying duties. As a result of its small dimension simplifies supply to cells, NovaIscB’s builders say it’s a promising candidate for growing gene therapies to deal with or stop illness.
The research was led by Feng Zhang, the James and Patricia Poitras Professor of Neuroscience at MIT who can also be an investigator on the McGovern Institute and the Howard Hughes Medical Institute, and a core member of the Broad Institute. Zhang and his workforce reported their open-access work this month within the journal Nature Biotechnology.
NovaIscB is derived from a bacterial DNA cutter that belongs to a household of proteins known as IscBs, which Zhang’s lab found in 2021. IscBs are a kind of OMEGA system, the evolutionary ancestors to Cas9, which is a part of the bacterial CRISPR system that Zhang and others have developed into highly effective genome-editing instruments. Like Cas9, IscB enzymes minimize DNA at websites specified by an RNA information. By reprogramming that information, researchers can redirect the enzymes to focus on sequences of their selecting.
IscBs had caught the workforce’s consideration not solely as a result of they share key options of CRISPR’s DNA-cutting Cas9, but additionally as a result of they’re a 3rd of its dimension. That may be a bonus for potential gene therapies: compact instruments are simpler to ship to cells, and with a small enzyme, researchers would have extra flexibility to tinker, probably including new functionalities with out creating instruments that have been too cumbersome for medical use.
From their preliminary research of IscBs, researchers in Zhang’s lab knew that some family members might minimize DNA targets in human cells. Not one of the bacterial proteins labored effectively sufficient to be deployed therapeutically, nevertheless: the workforce must modify an IscB to make sure it might edit targets in human cells effectively with out disturbing the remainder of the genome.
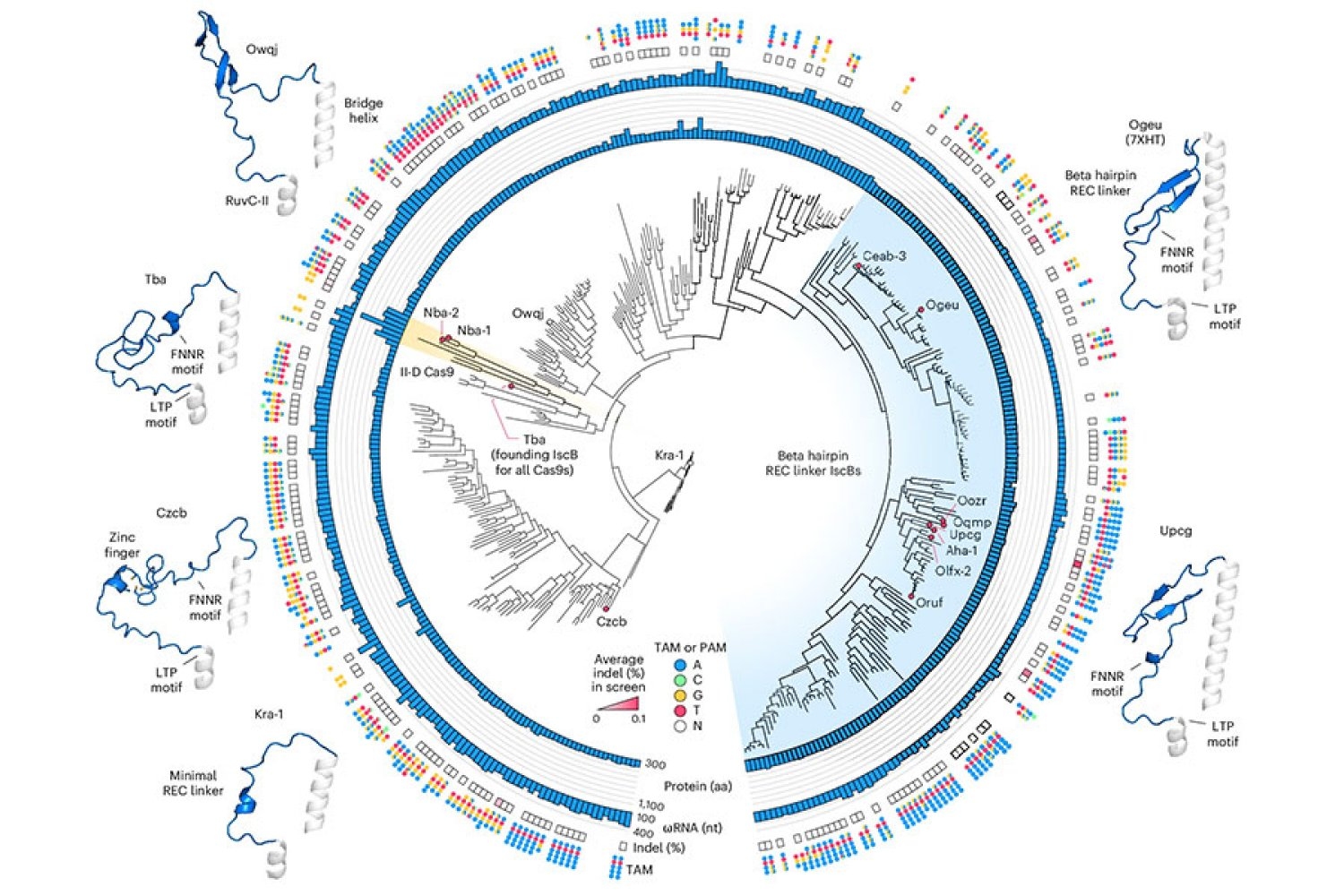
To start that engineering course of, Soumya Kannan, a graduate scholar in Zhang’s lab who’s now a junior fellow on the Harvard Society of Fellows, and postdoc Shiyou Zhu first looked for an IscB that might make good start line. They examined practically 400 totally different IscB enzymes that may be present in micro organism. Ten have been able to modifying DNA in human cells.
Even probably the most energetic of these would should be enhanced to make it a helpful genome modifying instrument. The problem can be rising the enzyme’s exercise, however solely on the sequences specified by its RNA information. If the enzyme turned extra energetic, however indiscriminately so, it could minimize DNA in unintended locations. “The secret’s to stability the development of each exercise and specificity on the similar time,” explains Zhu.
Zhu notes that bacterial IscBs are directed to their goal sequences by comparatively brief RNA guides, which makes it troublesome to limit the enzyme’s exercise to a particular a part of the genome. If an IscB might be engineered to accommodate an extended information, it could be much less prone to act on sequences past its meant goal.
To optimize IscB for human genome modifying, the workforce leveraged data that graduate scholar Han Altae-Tran, who’s now a postdoc on the College of Washington, had realized in regards to the range of bacterial IscBs and the way they developed. As an example, the researchers famous that IscBs that labored in human cells included a section they known as REC, which was absent in different IscBs. They suspected the enzyme would possibly want that section to work together with the DNA in human cells. Once they took a more in-depth have a look at the area, structural modeling instructed that by barely increasing a part of the protein, REC may also allow IscBs to acknowledge longer RNA guides.
Based mostly on these observations, the workforce experimented with swapping in components of REC domains from totally different IscBs and Cas9s, evaluating how every change impacted the protein’s perform. Guided by their understanding of how IscBs and Cas9s work together with each DNA and their RNA guides, the researchers made extra modifications, aiming to optimize each effectivity and specificity.
In the long run, they generated a protein they known as NovaIscB, which was over 100 instances extra energetic in human cells than the IscB that they had began with, and that had demonstrated good specificity for its targets.
Kannan and Zhu constructed and screened a whole lot of latest IscBs earlier than arriving at NovaIscB — and each change they made to the unique protein was strategic. Their efforts have been guided by their workforce’s information of IscBs’s pure evolution, in addition to predictions of how every alteration would influence the protein’s construction, made utilizing a man-made intelligence instrument known as AlphaFold2. In comparison with conventional strategies of introducing random modifications right into a protein and screening for his or her results, this rational engineering method drastically accelerated the workforce’s capability to establish a protein with the options they have been searching for.
The workforce demonstrated that NovaIscB is an efficient scaffold for quite a lot of genome modifying instruments. “It biochemically features very equally to Cas9, and that makes it straightforward to port over instruments that have been already optimized with the Cas9 scaffold,” Kannan says. With totally different modifications, the researchers used NovaIscB to exchange particular letters of the DNA code in human cells and to alter the exercise of focused genes.
Importantly, the NovaIscB-based instruments are compact sufficient to be simply packaged inside a single adeno-associated virus (AAV) — the vector mostly used to securely ship gene remedy to sufferers. As a result of they’re bulkier, instruments developed utilizing Cas9 can require a extra sophisticated supply technique.
Demonstrating NovaIscB’s potential for therapeutic use, Zhang’s workforce created a instrument known as OMEGAoff that provides chemical markers to DNA to dial down the exercise of particular genes. They programmed OMEGAoff to repress a gene concerned in ldl cholesterol regulation, then used AAV to ship the system to the livers of mice, resulting in lasting reductions in levels of cholesterol within the animals’ blood.
The workforce expects that NovaIscB can be utilized to focus on genome modifying instruments to most human genes, and stay up for seeing how different labs deploy the brand new expertise. In addition they hope others will undertake their evolution-guided method to rational protein engineering. “Nature has such range, and its programs have totally different benefits and drawbacks,” Zhu says. “By studying about that pure range, we are able to make the programs we are attempting to engineer higher and higher.”
This research was funded, partly, by the Ok. Lisa Yang and Hock E. Tan Heart for Molecular Therapeutics at MIT, Broad Institute Programmable Therapeutics Present Donors, Pershing Sq. Basis, William Ackman, Neri Oxman, the Phillips household, and J. and P. Poitras.

Scientists on the McGovern Institute for Mind Analysis at MIT and the Broad Institute of MIT and Harvard have re-engineered a compact RNA-guided enzyme they present in micro organism into an environment friendly, programmable editor of human DNA.
The protein they created, known as NovaIscB, may be tailored to make exact modifications to the genetic code, modulate the exercise of particular genes, or perform different modifying duties. As a result of its small dimension simplifies supply to cells, NovaIscB’s builders say it’s a promising candidate for growing gene therapies to deal with or stop illness.
The research was led by Feng Zhang, the James and Patricia Poitras Professor of Neuroscience at MIT who can also be an investigator on the McGovern Institute and the Howard Hughes Medical Institute, and a core member of the Broad Institute. Zhang and his workforce reported their open-access work this month within the journal Nature Biotechnology.
NovaIscB is derived from a bacterial DNA cutter that belongs to a household of proteins known as IscBs, which Zhang’s lab found in 2021. IscBs are a kind of OMEGA system, the evolutionary ancestors to Cas9, which is a part of the bacterial CRISPR system that Zhang and others have developed into highly effective genome-editing instruments. Like Cas9, IscB enzymes minimize DNA at websites specified by an RNA information. By reprogramming that information, researchers can redirect the enzymes to focus on sequences of their selecting.
IscBs had caught the workforce’s consideration not solely as a result of they share key options of CRISPR’s DNA-cutting Cas9, but additionally as a result of they’re a 3rd of its dimension. That may be a bonus for potential gene therapies: compact instruments are simpler to ship to cells, and with a small enzyme, researchers would have extra flexibility to tinker, probably including new functionalities with out creating instruments that have been too cumbersome for medical use.
From their preliminary research of IscBs, researchers in Zhang’s lab knew that some family members might minimize DNA targets in human cells. Not one of the bacterial proteins labored effectively sufficient to be deployed therapeutically, nevertheless: the workforce must modify an IscB to make sure it might edit targets in human cells effectively with out disturbing the remainder of the genome.
To start that engineering course of, Soumya Kannan, a graduate scholar in Zhang’s lab who’s now a junior fellow on the Harvard Society of Fellows, and postdoc Shiyou Zhu first looked for an IscB that might make good start line. They examined practically 400 totally different IscB enzymes that may be present in micro organism. Ten have been able to modifying DNA in human cells.
Even probably the most energetic of these would should be enhanced to make it a helpful genome modifying instrument. The problem can be rising the enzyme’s exercise, however solely on the sequences specified by its RNA information. If the enzyme turned extra energetic, however indiscriminately so, it could minimize DNA in unintended locations. “The secret’s to stability the development of each exercise and specificity on the similar time,” explains Zhu.
Zhu notes that bacterial IscBs are directed to their goal sequences by comparatively brief RNA guides, which makes it troublesome to limit the enzyme’s exercise to a particular a part of the genome. If an IscB might be engineered to accommodate an extended information, it could be much less prone to act on sequences past its meant goal.
To optimize IscB for human genome modifying, the workforce leveraged data that graduate scholar Han Altae-Tran, who’s now a postdoc on the College of Washington, had realized in regards to the range of bacterial IscBs and the way they developed. As an example, the researchers famous that IscBs that labored in human cells included a section they known as REC, which was absent in different IscBs. They suspected the enzyme would possibly want that section to work together with the DNA in human cells. Once they took a more in-depth have a look at the area, structural modeling instructed that by barely increasing a part of the protein, REC may also allow IscBs to acknowledge longer RNA guides.
Based mostly on these observations, the workforce experimented with swapping in components of REC domains from totally different IscBs and Cas9s, evaluating how every change impacted the protein’s perform. Guided by their understanding of how IscBs and Cas9s work together with each DNA and their RNA guides, the researchers made extra modifications, aiming to optimize each effectivity and specificity.
In the long run, they generated a protein they known as NovaIscB, which was over 100 instances extra energetic in human cells than the IscB that they had began with, and that had demonstrated good specificity for its targets.
Kannan and Zhu constructed and screened a whole lot of latest IscBs earlier than arriving at NovaIscB — and each change they made to the unique protein was strategic. Their efforts have been guided by their workforce’s information of IscBs’s pure evolution, in addition to predictions of how every alteration would influence the protein’s construction, made utilizing a man-made intelligence instrument known as AlphaFold2. In comparison with conventional strategies of introducing random modifications right into a protein and screening for his or her results, this rational engineering method drastically accelerated the workforce’s capability to establish a protein with the options they have been searching for.
The workforce demonstrated that NovaIscB is an efficient scaffold for quite a lot of genome modifying instruments. “It biochemically features very equally to Cas9, and that makes it straightforward to port over instruments that have been already optimized with the Cas9 scaffold,” Kannan says. With totally different modifications, the researchers used NovaIscB to exchange particular letters of the DNA code in human cells and to alter the exercise of focused genes.
Importantly, the NovaIscB-based instruments are compact sufficient to be simply packaged inside a single adeno-associated virus (AAV) — the vector mostly used to securely ship gene remedy to sufferers. As a result of they’re bulkier, instruments developed utilizing Cas9 can require a extra sophisticated supply technique.
Demonstrating NovaIscB’s potential for therapeutic use, Zhang’s workforce created a instrument known as OMEGAoff that provides chemical markers to DNA to dial down the exercise of particular genes. They programmed OMEGAoff to repress a gene concerned in ldl cholesterol regulation, then used AAV to ship the system to the livers of mice, resulting in lasting reductions in levels of cholesterol within the animals’ blood.
The workforce expects that NovaIscB can be utilized to focus on genome modifying instruments to most human genes, and stay up for seeing how different labs deploy the brand new expertise. In addition they hope others will undertake their evolution-guided method to rational protein engineering. “Nature has such range, and its programs have totally different benefits and drawbacks,” Zhu says. “By studying about that pure range, we are able to make the programs we are attempting to engineer higher and higher.”
This research was funded, partly, by the Ok. Lisa Yang and Hock E. Tan Heart for Molecular Therapeutics at MIT, Broad Institute Programmable Therapeutics Present Donors, Pershing Sq. Basis, William Ackman, Neri Oxman, the Phillips household, and J. and P. Poitras.

Scientists on the McGovern Institute for Mind Analysis at MIT and the Broad Institute of MIT and Harvard have re-engineered a compact RNA-guided enzyme they present in micro organism into an environment friendly, programmable editor of human DNA.
The protein they created, known as NovaIscB, may be tailored to make exact modifications to the genetic code, modulate the exercise of particular genes, or perform different modifying duties. As a result of its small dimension simplifies supply to cells, NovaIscB’s builders say it’s a promising candidate for growing gene therapies to deal with or stop illness.
The research was led by Feng Zhang, the James and Patricia Poitras Professor of Neuroscience at MIT who can also be an investigator on the McGovern Institute and the Howard Hughes Medical Institute, and a core member of the Broad Institute. Zhang and his workforce reported their open-access work this month within the journal Nature Biotechnology.
NovaIscB is derived from a bacterial DNA cutter that belongs to a household of proteins known as IscBs, which Zhang’s lab found in 2021. IscBs are a kind of OMEGA system, the evolutionary ancestors to Cas9, which is a part of the bacterial CRISPR system that Zhang and others have developed into highly effective genome-editing instruments. Like Cas9, IscB enzymes minimize DNA at websites specified by an RNA information. By reprogramming that information, researchers can redirect the enzymes to focus on sequences of their selecting.
IscBs had caught the workforce’s consideration not solely as a result of they share key options of CRISPR’s DNA-cutting Cas9, but additionally as a result of they’re a 3rd of its dimension. That may be a bonus for potential gene therapies: compact instruments are simpler to ship to cells, and with a small enzyme, researchers would have extra flexibility to tinker, probably including new functionalities with out creating instruments that have been too cumbersome for medical use.
From their preliminary research of IscBs, researchers in Zhang’s lab knew that some family members might minimize DNA targets in human cells. Not one of the bacterial proteins labored effectively sufficient to be deployed therapeutically, nevertheless: the workforce must modify an IscB to make sure it might edit targets in human cells effectively with out disturbing the remainder of the genome.
To start that engineering course of, Soumya Kannan, a graduate scholar in Zhang’s lab who’s now a junior fellow on the Harvard Society of Fellows, and postdoc Shiyou Zhu first looked for an IscB that might make good start line. They examined practically 400 totally different IscB enzymes that may be present in micro organism. Ten have been able to modifying DNA in human cells.
Even probably the most energetic of these would should be enhanced to make it a helpful genome modifying instrument. The problem can be rising the enzyme’s exercise, however solely on the sequences specified by its RNA information. If the enzyme turned extra energetic, however indiscriminately so, it could minimize DNA in unintended locations. “The secret’s to stability the development of each exercise and specificity on the similar time,” explains Zhu.
Zhu notes that bacterial IscBs are directed to their goal sequences by comparatively brief RNA guides, which makes it troublesome to limit the enzyme’s exercise to a particular a part of the genome. If an IscB might be engineered to accommodate an extended information, it could be much less prone to act on sequences past its meant goal.
To optimize IscB for human genome modifying, the workforce leveraged data that graduate scholar Han Altae-Tran, who’s now a postdoc on the College of Washington, had realized in regards to the range of bacterial IscBs and the way they developed. As an example, the researchers famous that IscBs that labored in human cells included a section they known as REC, which was absent in different IscBs. They suspected the enzyme would possibly want that section to work together with the DNA in human cells. Once they took a more in-depth have a look at the area, structural modeling instructed that by barely increasing a part of the protein, REC may also allow IscBs to acknowledge longer RNA guides.
Based mostly on these observations, the workforce experimented with swapping in components of REC domains from totally different IscBs and Cas9s, evaluating how every change impacted the protein’s perform. Guided by their understanding of how IscBs and Cas9s work together with each DNA and their RNA guides, the researchers made extra modifications, aiming to optimize each effectivity and specificity.
In the long run, they generated a protein they known as NovaIscB, which was over 100 instances extra energetic in human cells than the IscB that they had began with, and that had demonstrated good specificity for its targets.
Kannan and Zhu constructed and screened a whole lot of latest IscBs earlier than arriving at NovaIscB — and each change they made to the unique protein was strategic. Their efforts have been guided by their workforce’s information of IscBs’s pure evolution, in addition to predictions of how every alteration would influence the protein’s construction, made utilizing a man-made intelligence instrument known as AlphaFold2. In comparison with conventional strategies of introducing random modifications right into a protein and screening for his or her results, this rational engineering method drastically accelerated the workforce’s capability to establish a protein with the options they have been searching for.
The workforce demonstrated that NovaIscB is an efficient scaffold for quite a lot of genome modifying instruments. “It biochemically features very equally to Cas9, and that makes it straightforward to port over instruments that have been already optimized with the Cas9 scaffold,” Kannan says. With totally different modifications, the researchers used NovaIscB to exchange particular letters of the DNA code in human cells and to alter the exercise of focused genes.
Importantly, the NovaIscB-based instruments are compact sufficient to be simply packaged inside a single adeno-associated virus (AAV) — the vector mostly used to securely ship gene remedy to sufferers. As a result of they’re bulkier, instruments developed utilizing Cas9 can require a extra sophisticated supply technique.
Demonstrating NovaIscB’s potential for therapeutic use, Zhang’s workforce created a instrument known as OMEGAoff that provides chemical markers to DNA to dial down the exercise of particular genes. They programmed OMEGAoff to repress a gene concerned in ldl cholesterol regulation, then used AAV to ship the system to the livers of mice, resulting in lasting reductions in levels of cholesterol within the animals’ blood.
The workforce expects that NovaIscB can be utilized to focus on genome modifying instruments to most human genes, and stay up for seeing how different labs deploy the brand new expertise. In addition they hope others will undertake their evolution-guided method to rational protein engineering. “Nature has such range, and its programs have totally different benefits and drawbacks,” Zhu says. “By studying about that pure range, we are able to make the programs we are attempting to engineer higher and higher.”
This research was funded, partly, by the Ok. Lisa Yang and Hock E. Tan Heart for Molecular Therapeutics at MIT, Broad Institute Programmable Therapeutics Present Donors, Pershing Sq. Basis, William Ackman, Neri Oxman, the Phillips household, and J. and P. Poitras.

Scientists on the McGovern Institute for Mind Analysis at MIT and the Broad Institute of MIT and Harvard have re-engineered a compact RNA-guided enzyme they present in micro organism into an environment friendly, programmable editor of human DNA.
The protein they created, known as NovaIscB, may be tailored to make exact modifications to the genetic code, modulate the exercise of particular genes, or perform different modifying duties. As a result of its small dimension simplifies supply to cells, NovaIscB’s builders say it’s a promising candidate for growing gene therapies to deal with or stop illness.
The research was led by Feng Zhang, the James and Patricia Poitras Professor of Neuroscience at MIT who can also be an investigator on the McGovern Institute and the Howard Hughes Medical Institute, and a core member of the Broad Institute. Zhang and his workforce reported their open-access work this month within the journal Nature Biotechnology.
NovaIscB is derived from a bacterial DNA cutter that belongs to a household of proteins known as IscBs, which Zhang’s lab found in 2021. IscBs are a kind of OMEGA system, the evolutionary ancestors to Cas9, which is a part of the bacterial CRISPR system that Zhang and others have developed into highly effective genome-editing instruments. Like Cas9, IscB enzymes minimize DNA at websites specified by an RNA information. By reprogramming that information, researchers can redirect the enzymes to focus on sequences of their selecting.
IscBs had caught the workforce’s consideration not solely as a result of they share key options of CRISPR’s DNA-cutting Cas9, but additionally as a result of they’re a 3rd of its dimension. That may be a bonus for potential gene therapies: compact instruments are simpler to ship to cells, and with a small enzyme, researchers would have extra flexibility to tinker, probably including new functionalities with out creating instruments that have been too cumbersome for medical use.
From their preliminary research of IscBs, researchers in Zhang’s lab knew that some family members might minimize DNA targets in human cells. Not one of the bacterial proteins labored effectively sufficient to be deployed therapeutically, nevertheless: the workforce must modify an IscB to make sure it might edit targets in human cells effectively with out disturbing the remainder of the genome.
To start that engineering course of, Soumya Kannan, a graduate scholar in Zhang’s lab who’s now a junior fellow on the Harvard Society of Fellows, and postdoc Shiyou Zhu first looked for an IscB that might make good start line. They examined practically 400 totally different IscB enzymes that may be present in micro organism. Ten have been able to modifying DNA in human cells.
Even probably the most energetic of these would should be enhanced to make it a helpful genome modifying instrument. The problem can be rising the enzyme’s exercise, however solely on the sequences specified by its RNA information. If the enzyme turned extra energetic, however indiscriminately so, it could minimize DNA in unintended locations. “The secret’s to stability the development of each exercise and specificity on the similar time,” explains Zhu.
Zhu notes that bacterial IscBs are directed to their goal sequences by comparatively brief RNA guides, which makes it troublesome to limit the enzyme’s exercise to a particular a part of the genome. If an IscB might be engineered to accommodate an extended information, it could be much less prone to act on sequences past its meant goal.
To optimize IscB for human genome modifying, the workforce leveraged data that graduate scholar Han Altae-Tran, who’s now a postdoc on the College of Washington, had realized in regards to the range of bacterial IscBs and the way they developed. As an example, the researchers famous that IscBs that labored in human cells included a section they known as REC, which was absent in different IscBs. They suspected the enzyme would possibly want that section to work together with the DNA in human cells. Once they took a more in-depth have a look at the area, structural modeling instructed that by barely increasing a part of the protein, REC may also allow IscBs to acknowledge longer RNA guides.
Based mostly on these observations, the workforce experimented with swapping in components of REC domains from totally different IscBs and Cas9s, evaluating how every change impacted the protein’s perform. Guided by their understanding of how IscBs and Cas9s work together with each DNA and their RNA guides, the researchers made extra modifications, aiming to optimize each effectivity and specificity.
In the long run, they generated a protein they known as NovaIscB, which was over 100 instances extra energetic in human cells than the IscB that they had began with, and that had demonstrated good specificity for its targets.
Kannan and Zhu constructed and screened a whole lot of latest IscBs earlier than arriving at NovaIscB — and each change they made to the unique protein was strategic. Their efforts have been guided by their workforce’s information of IscBs’s pure evolution, in addition to predictions of how every alteration would influence the protein’s construction, made utilizing a man-made intelligence instrument known as AlphaFold2. In comparison with conventional strategies of introducing random modifications right into a protein and screening for his or her results, this rational engineering method drastically accelerated the workforce’s capability to establish a protein with the options they have been searching for.
The workforce demonstrated that NovaIscB is an efficient scaffold for quite a lot of genome modifying instruments. “It biochemically features very equally to Cas9, and that makes it straightforward to port over instruments that have been already optimized with the Cas9 scaffold,” Kannan says. With totally different modifications, the researchers used NovaIscB to exchange particular letters of the DNA code in human cells and to alter the exercise of focused genes.
Importantly, the NovaIscB-based instruments are compact sufficient to be simply packaged inside a single adeno-associated virus (AAV) — the vector mostly used to securely ship gene remedy to sufferers. As a result of they’re bulkier, instruments developed utilizing Cas9 can require a extra sophisticated supply technique.
Demonstrating NovaIscB’s potential for therapeutic use, Zhang’s workforce created a instrument known as OMEGAoff that provides chemical markers to DNA to dial down the exercise of particular genes. They programmed OMEGAoff to repress a gene concerned in ldl cholesterol regulation, then used AAV to ship the system to the livers of mice, resulting in lasting reductions in levels of cholesterol within the animals’ blood.
The workforce expects that NovaIscB can be utilized to focus on genome modifying instruments to most human genes, and stay up for seeing how different labs deploy the brand new expertise. In addition they hope others will undertake their evolution-guided method to rational protein engineering. “Nature has such range, and its programs have totally different benefits and drawbacks,” Zhu says. “By studying about that pure range, we are able to make the programs we are attempting to engineer higher and higher.”
This research was funded, partly, by the Ok. Lisa Yang and Hock E. Tan Heart for Molecular Therapeutics at MIT, Broad Institute Programmable Therapeutics Present Donors, Pershing Sq. Basis, William Ackman, Neri Oxman, the Phillips household, and J. and P. Poitras.













